Author: Sarah F Roberts
Abstract
In Leptothorax acervorum and several related species virgin queens return to their maternal nest after an unsuccessful mating season and shed their wings after which they are unlikely to ever mate. These unmated queens are known to have a different behavioural trope to the reproductive queens in the colony and are sometimes described as acting like workers. To test if unmated queens do fulfil the same functional role in a colony as workers colonies of L. acervorum were collected from Thetford forest in Norfolk and kept in artificial nests while the workers, unmated queens and mated queens were examined in several different situations. This allowed comparison of each caste’s behaviour in a functioning nest, in an unfamiliar environment, when their nest is disturbed, and when presented with an aggressor or conspecific from another colony. Unmated queens were found to be significantly different to mated queens in their behaviours, often more so than workers were. However unmated queens were not significantly similar to workers, except in that they all fled when their nest was disturbed, indicating their behavioural tropes and therefore functional roles in a colony are not exactly the same though they do overlap in many ways.
Introduction
It is common in species of Leptothorax to be capable of both polygyny and monogyny as is the case with Leptothorax acervorum (Gill, Arce, Keller, & Hammond, 2009; Thomas M. Alloway, 1982). Despite this social polymorphism, colonies from all populations contain dealate virgin queens alongside mated queens and workers. Each of these castes performs a functional role in the colony; the role of reproductive queens is to produce fertilised eggs, the role of workers is to care for brood items and meet the resource requirements of the colony by foraging and feeding others by trophallaxis (Trettin, Seyferth, & Heinze, 2014). The role of unmated queens is less understood however.
Colonies of L. acervorum often contain fewer than 100 workers and live in decaying twigs where they have made a nest by excavating holes bored into the wood by beetle larvae (Kuhbandner, Modlmeier, & Foitzik, 2014). Virgin queens mate in late summer or early autumn during a nuptial flight (Heinze, Holldobler, & Trenkle, 1995). In English colonies the majority of queens then return to their originally colony thus maintaining polygynous colonies (Felke & Buschinger, 1999). Queens shed their wings within a day after mating or at the end of the mating season if unmated (Trettin et al., 2014). Mated queens then undergo reproductive development either immediately after mating or during spring after hibernation when physiological, morphological and behavioural changes occur. The surface hydrocarbons of newly mated queens have changed by the time they return to their maternal nest (Oppelt & Heinze, 2009; Ortius & Heinze, 1999), while the composition of secretions from the Dufour gland change so that they are no longer as attractive to males (Eliyahu, Ross, Haight, Keller, & Liebig, 2011; Oberstadt & Heinze, 2003). Later the ovarioles develop becoming longer and causing the abdomen to become physogastric (Gill et al., 2009), following this eggs will begin to develop in the ovarioles and the ovarioles will secrete exocrine hormones (Kuhbandner et al., 2014), after which the volume of the brain will diminish (Julian & Gronenberg, 2002). After mating queens also become less exploratory, less active and begin to seek dark places rather than light and stop seeking higher ground (Bernadou & Heinze, 2013). These behavioural differences are believed to arise due to the differences in brain volume in areas associated with behavioural repertoire size and acuity of some senses such as sight which mated queens are unlikely to need inside the darkened nest chamber. That such changes after mating can be attributed to loss of brain tissue explains why they are irreversible (Ruppell, Schaffler, & Holldobler, 2002).
These changes not only mean that the queens behave differently but that others behave differently towards them particularly because of the change in surface hydrocarbons and other chemical cues which identify them as fertile (Coston, Gill, & Hammond, 2011; Ortius & Heinze, 1999). In monogynous colonies of L. acervorum the newly mated queens are often attacked and sometimes expelled from their maternal colony while unmated queens never are (Felke & Buschinger, 1999).
Being polygynous has clear benefits to a colony; by keeping several mated queens the colony ensures that if one dies there are others to take her place. However it is possible to have too many reproducing queens and virgin queens are believed to be dealated by workers in some cases to prevent them mating because too many reproducing queens would put too high a resource demand on the colony for workers to meet and potentially reduce the lifespan of all queens (Hammond, Bruford, & Bourke, 2003; Heinze & Oberstadt, 2003; Schrempf, Cremer, & Heinze, 2011). There could be other reasons why unmated queens are dealated which is not to do with resource costs however colonies also produce fewer queens when there are more reproducing queens in a nest suggesting they do react to the number of reproducing queens present (Heinze, Lipski, Schlehmeyer, & Holldobler, 1995). It has also been shown that workers do not influence the reproduction of queens based on relatedness so unmated queens would not attempt to remove the wings of an unmated female due to low relatedness (Friend & Bourke, 2012; Gill & Hammond, 2011c; Hammond, Bruford, & Bourke, 2006).
However if by mating they could be detrimental to the colony, there must be a reason for colonies to risk this unfavourable effect and keep unmated queens rather than expelling them from the colony. The fact that they are kept suggests unmated queens must serve some functional role in the colony and the behavioural differences that have been observed between mated and unmated individuals in several Leptothorax species including L. acervorum suggest that this functional role is different to that of mated queens. Ito (2005) suggests unmated queens fulfil the same function as workers but bases this only on similar aggression towards low ranking mated queens in functionally monogynous colonies, which workers are known to do in order to maintain reproductive dominance by one queen. However they could perform an additional benefit to colonies in the same way that subordinate queens do in monogynous colonies; increasing the biomass production of reproductive queens (Heinze & Oberstadt, 2003). If unmated queens do fulfil the same functional role as workers, they would likely have the same behavioural trope as workers. If they have a different behavioural trope this would suggest unmated queens perform a different functional role in the colony.
This study will examine the behaviour of dealate virgin queens in relation to that of workers and reproductive mated queens in a variety of situations to determine how the behaviour of non-mated L. acervorum queens differs from mated queens and whether it is similar enough to that of workers to suggest they fulfil the same role.
Materials and Methods
Colony collection
8 colonies of Leptothorax acervorum were collected from Thetford forest in Norfolk on 13 October 2014. It was overcast and raining throughout with little wind during the collection period between 12:45 and 16:00. Colonies were collected from three different areas near Santon Downham referred to as SD1, SD2 and SD3 yielding 5, 2 and 1 colonies respectively. When a colony was found by breaking open a twig, the pieces of the hollowed out twig were kept together in plastic zip lock bags, then the bag sealed and marked with the area reference e.g. SD1. Since all pieces of a colony’s twig were collected and kept together as done in previous studies of this species (Gill & Hammond, 2011c) it is likely that the majority of each colony and all mated queens were collected.
Artificial nests and colony care
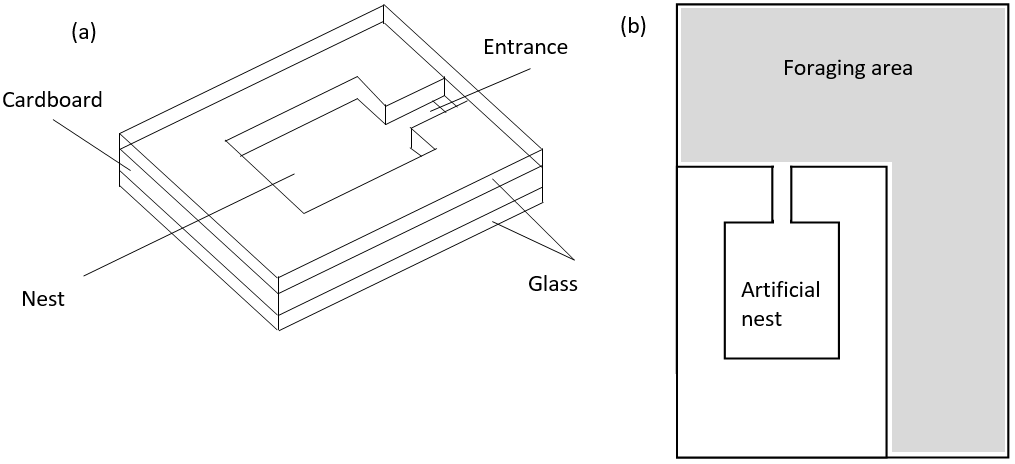
Artificial nests were made by cutting an 2mm wide entrance tunnel and 30mm x 35mm nest chamber out of thin cardboard, sandwiching this between two 55mm x 80mm glass slides and fixing the pieces together using sellotape (see figure 1 (a)) (Bourke, 1991). Each artificial nest was then placed inside an 80mm x 125mm clear plastic container, the bottom of which was half filled with plaster of Paris and the sides coated with fluon to hinder ants climbing up the sides (see figure 1 (b)). The twigs were then opened and each colony transferred to its own container, ensuring all queens, workers and brood items found were transferred safely using flexible Storkbill fine pointed forceps. Nests were labelled with the relevant colony’s area reference and a small amount of water (approx. 5ml) was allowed to soak into the plaster of Paris to maintain humidity in each container. All colonies were transferred between 12:00 and 15:00 two days after initial collection.
Colonies boxes were cleaned of debris from the twigs once the ants had collected all brood items and taken them into artificial nest chamber. If colonies had not moved into the artificial nest the colony box was put under bright light and the artificial nest covered with a piece of card so the only shaded area was inside the nest chamber. L. acervorum colonies were fed with a section (approx. 5mm) of mealworm and honey water soaked into a cotton ball (approx. 10mm in diameter) and watered to maintain humidity every Monday and Thursday (Gill et al., 2009). All colonies were kept in a Versatile Environmental Test Chamber (Sanyo MLR-351H) cycling in an autumn setting between 10°C and 20°C and 70% and 80% humidity whenever they were not being experimented on. Each colony was allowed five days to acclimatise to the artificial nests and foraging area before marking and at least a further two days after marking before experimentation began.
Census and marking
A census was performed for each colony counting the number of queens, workers larvae and eggs, and the colonies labelled SR.1 to SR.8 for future reference. At least 1 queen was present in each colony except SR.5 giving a total of 16 queens. Deceased individuals were excluded for example two queen ants which were missing their head and abdomen respectively were not counted in the census of SR.7. Queens were identified by their thoraces, which appear as several distinguishable segments on queens with wider sides due to wing muscles and small protrusions where wings were attached prior to dealation and as a single piece with few distinctive features on workers (Heinze & Buschinger, 1987). Marking methods were trialled on workers prior to marking queens resulting in preferred use of wire marking over painting of gasters or thoraces since paint could glue the ants body parts together if applied poorly and can be cleaned off to some degree by nest mates (Friend & Bourke, 2012). Queens were marked using a combination of wires tied around the pronotum and gaster to allow distinction of different queens within a colony. 5 workers from each of SR.3, SR.5 and SR.8 were similarly marked as these colonies had fewest queens so worker markings would not be as easily confused with queen markings.
Normal nest behaviour experiment
Each colony was recorded using a Logitech HD Pro webcam at 720p resolution between 10:00 and 11:00, 13:00 and 14:00 and 16:00 and 17:00 to provide a sample of behaviours at different times of day in case the individuals’ behaviour was affected by their circadian rhythms. The highest available resolution was used as wire markings distinguishing individuals could not be made out at lower resolutions. The recordings consisted of a video of the nest chamber of each colony lasting 20 minutes as previous studies suggest a cyclic pattern of the individual’s normal behaviours should be exhibited approximately every 20 minutes (Boi, Couzin, Del Buono, Franks, & Britton, 1999; Hatcher, Tofts, & Franks, 1992). These videos were used to make a focus sample of marked individuals recording the total time spent by each of the 16 queens and 15 workers resting, grooming themselves, grooming others including brood items, being groomed, bullying others, being bullied, foraging, and moving around the nest chamber. Resting was defined as remaining motionless for 3 seconds or more and foraging as any time spent outside the nest chamber. Colonies to be tested each day were randomly chosen using an online random number generator.
Nest disturbance experiment
The upper glass slide forming the nest was removed so that the response of marked individuals to such disturbance could be ascertained in terms of whether they left the exposed nest chamber or stayed and whether they collected or ignored brood items. The artificial nest was again recorded with the Logitech webcam at 720p resolution for 10 minutes between 10:00 and 11:00, 13:00 and 14:00 and 16:00 and 17:00 to give three repeats for each individual and colonies to be tested chosen randomly as before. This produced binary (positive or negative) scores for both behaviours, “flee” and “collect brood” 10 minutes was regarded as sufficient time as most, if not all, brood items and individuals had been removed from the nest chamber in this time.
New environment experiment
A random unoccupied colony box was used from a pool of spare colony boxes then filmed with the Logitech webcam as a randomly chosen marked individual from the colony being tested that day was placed in the box and covered by a transparent lid with a 55mm x 55mm piece of card fixed to one corner creating an area of shade. Each 30 minute video was cut into five consecutive 6 minute periods. For each of an individual’s 6 minute periods the time spent in the non-shaded “light” area and the number of transitions between the light and shaded areas was recorded producing continuous data for both time spent light and the number of transition made between the light and shaded areas.
The new environment experiment and the following two experiments were all performed on the same day for all queens and marked workers of a given colony following their morning nest disturbance experiment. Although performing experiments back to back may affect behaviour all individuals experienced this same treatment and the alternative of repeatedly disturbing the nest to retrieve individuals may also affect behaviour. Furthermore this enabled behaviour in a wider variety of situations to be studied as it streamlined the experimentation process so more experiments could be carried out within the time constraints.
Antennal probing experiment
Again a random unoccupied colony box was used to contain the individual being tested while fine forceps were used to gently touch or stroke the individual’s antennae such that the individual is not physically injured. The responses were viewed down a dissecting microscope and recorded as fight if the individual showed aggressive behaviour, such as rearing, mandible spreading or biting the forceps, or flee if the individual turned around and moved away from the forceps. This procedure was repeated five times for each individual and a minimum interval of two minutes was given between repeats.
Frozen worker interaction experiment
A petri dish was randomly chosen from a pool of available 55mm diameter petri dishes with fluon coated sides and the individual being tested was placed in the dish with a frozen worker from a colony with a different area reference to the individual being tested. For example an SR.2 queen collected in SD1 would be placed with a frozen worker from a colony collected in SD2 or SD3. This was done to ensure maximum genetic difference between the two individuals so they would not be treated as a nest mate, since a colony from the same area could have been a daughter colony formed by a newly mated queen with workers from the maternal colony and may still be recognised by individuals from the maternal colony or vice versa. Workers used to induce responses were frozen so that they could not injure or kill the individuals being tested which might be needed for later nest disturbance experiments but would still cause a response (Modlmeier & Foitzik, 2011). The smallest available petri dishes were used to increase the frequency with which marked individuals encountered frozen workers since size of containment has been shown to have no effect on aggression behaviour (Roulston, Buczkowski, & Silverman, 2003). The first five responses when marked individuals encountered frozen workers were recorded as flee, ignore or fight, scored as 0, 1 and 2 respectively. Flee was given the lowest score as it was purely defensive and avoided aggression, ignore was scored in between the other two responses as it was a neutral response neither avoiding aggression from others nor a display of aggression in itself and fight was scored highest as it was recorded for any displays of aggression including biting, dragging, rearing and mandible spreading.
Dissections
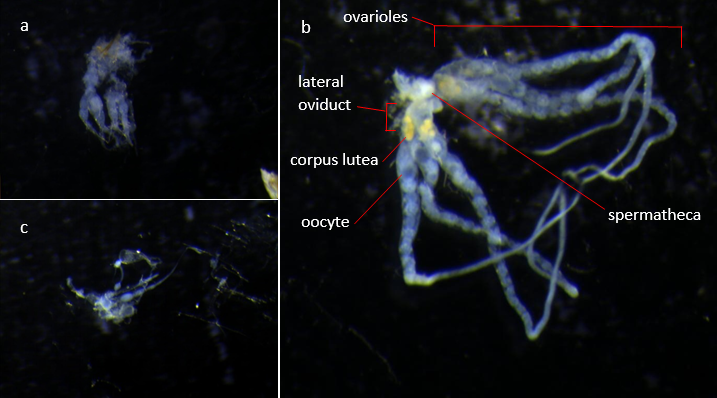
All colonies were frozen at -20˚C for a week prior to dissection to ensure all individuals were dead. Dissections were done under a dissecting microscope using fine forceps to hold the individual under water and reach under the final tergite of the gaster and pull the reproductive organs out by the ovipositor. Mated queens were identified primarily by their spermathecae being opaque due to the presence of sperm, with supporting evidence from ovariole development and the presence of eggs and corpus lutea (Gill et al., 2009). The reproductive organs were cleaned of tracheoles and other undesired tissues before recording mated status and ovariole development in qualitative terms. Dissected reproductive organs were then photographed down the microscope using Xli Cap software (XL Imaging Ltd, 2006). These images, examples of which are shown in figure 2, were then used to measure ovariole and oocyte lengths as a proportion of lateral oviduct length since these lateral oviducts were intact and relatively consistent in length throughout all individuals. These standardised measurements were based on length in pixels calculated using imageJ (Rasband, 1997-2014).
Analyses
Measurements for each individual were averaged for each experiment to avoid pseudo-replication of data; binary data for nest disturbance and antennal probing experiments were averaged by mode to keep the data sets in binary format, while all other data sets were averaged by mean. All statistical analyses were then carried out using Minitab (Minitab Inc, 2010). Due to qualitative observations of behavioural differences during data collection, in particular they appeared less active, workers from the queenless colony, SR.5, were examined prior to further analyses to determine whether their behaviour was an accurate representation of worker behaviour. Workers from SR.5 were compared to the other 10 marked workers using Principal Component Analysis (PCA) which showed the behaviour of SR.5 workers formed a distinct cluster outside the range of behaviour of other workers. Discriminant function analysis (DFA) also identified SR.5 as a distinct group while the SR.3 and SR.8 workers were incorrectly grouped with each other’s colonies (P = 0.867). Reasons why their behaviour may differ from that of other workers was also found in the literature; for example the reduced number of eggs and larvae in SR.5 compared to other colonies and there being fewer interactions between workers as there were fewer workers could cause an overall reduction in the activity of workers (Cole & Cheshire, 1996; Cole & Hoeg, 1996). Because of this difference in behaviour these five workers were excluded from all further behavioural analyses.
The collated data for normal nest behaviour was examined using PCA and DFA to obtain graphical and numerical representations respectively of similarity in behaviour between the three castes based on all areas of behaviour described. Binary data sets for “flee” and “collect brood” behaviours in nest disturbance and responses in antennal probing were compared using Fisher’s exact test as the data sets were too small for reliable use of χ2 (Bower, 2003). The remaining continuous data sets for the new environment and frozen worker experiments were compared using analysis of variance (ANOVA) followed by a post-hoc Tukey’s test.
The continuous data sets which were found to be statistically significant in difference between mated and non-mated queens were analysed using a Pearson correlation against standardised ovariole length to assess whether the change in behaviour is correlated to ovarian development or otherwise linked more directly to other factors resulting from mating.
Results
Dissections
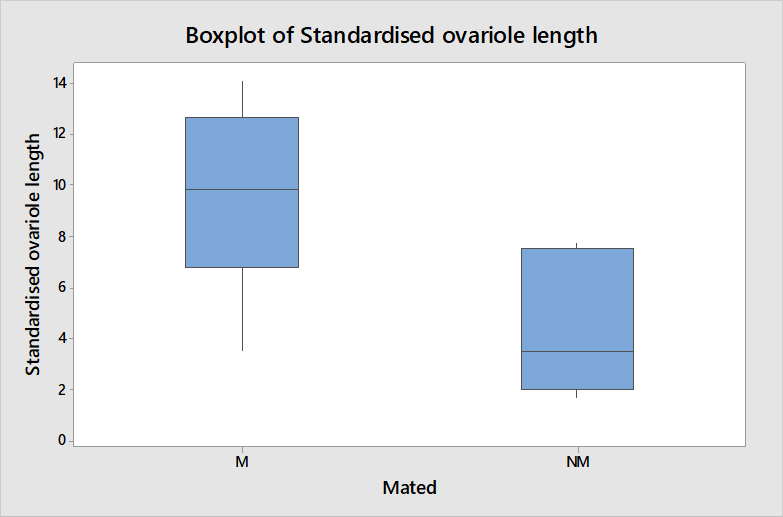
Table 1 shows a clear difference in that the mated queens were all producing eggs and non-mated queens were not. The length of ovarioles shown in figure 3 is also significantly different between mated and non-mated queens when compared using a t-test (t = 3.22, df = 12, P = 0.007) thus it is an appropriate measure of physiological change occurring in queens after mating to be used in further analysis.
New Environment
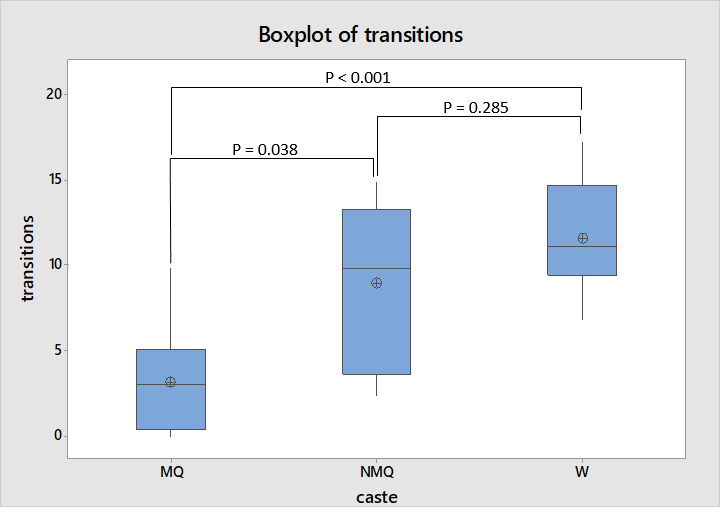
In the new environment experiment there was greatest difference in the number of transitions made by individuals between light and shaded areas with the average number of transitions made by mated queens being much lower than that of the other two castes as shown in figure 4. ANOVA followed by Tukey’s test reflects this, indicating that mated queens make a significantly lower number of transitions compared to both non-mated queens and workers, while workers and non-mated queens are not dissimilar to each other (F = 11.31, df = 2, 20, P = 0.001).
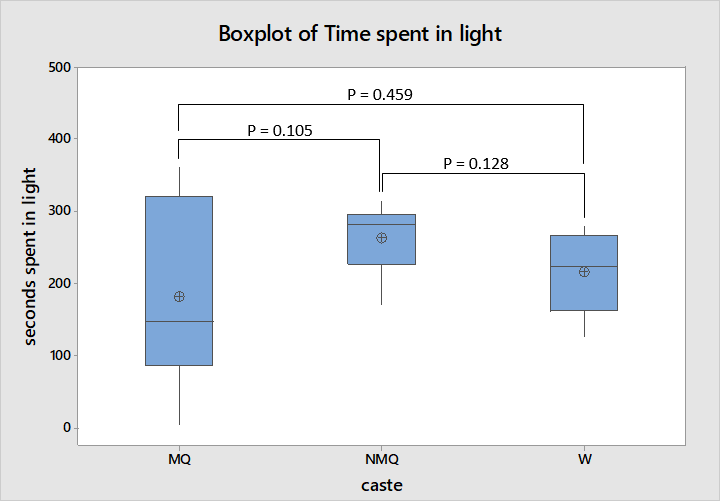
The amount of time spent in the light was most variable for mated queens ranging from 5 seconds to all 360 seconds of the experiment as shown in figure 5. The interquartile ranges of all three castes overlap suggesting a lack of dissimilarity confirmed by ANOVA; neither mated and non-mated queens, workers and mated queens or workers and non-mated queens showed statistically significant dissimilarity (F = 1.52, df = 2, 22, P = 0.243). Though this may be a poor indication of difference in preferences for light or dark areas as several mated queens never found the shaded area.
Frozen Worker Interactions
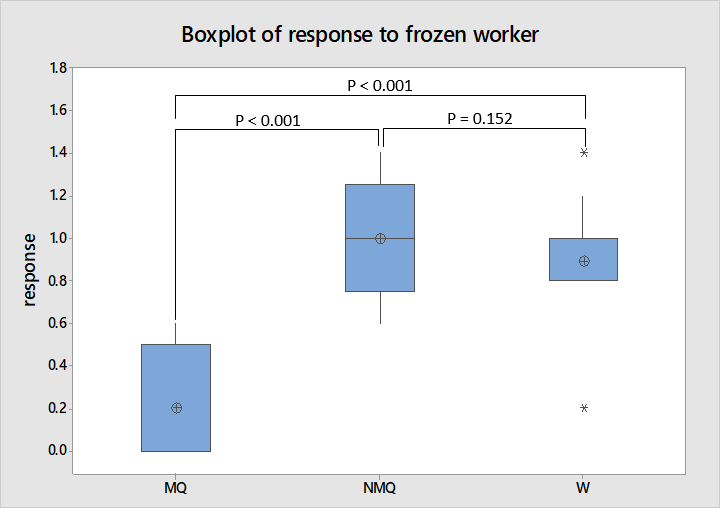
As shown in figure 6 the behavioural scores for frozen worker interactions have no overlap between mated and non-mated queens or workers. This is supported by ANOVA where the subsequent Tukey’s test identified a significant difference in behaviour towards non-nest mate individuals between mated and non-mated queens and mated queens and workers (F = 21.53, df = 2, 25, P < 0.001). However as with the new environment experiment non-mated queens were not significantly different in their behaviour to workers which is reflected in the box plot where the interquartile ranges of worker and non-mated queen responses overlap.
Antennal Probing
The Fisher’s exact tests showed a statistically significant difference in the aggression shown by mated and non-mated queens (P = 0.0440). However since workers had an intermediate proportion of individuals whose normal response to antennal probing was to fight rather than flee compared to mated and non-mated queens, there was no dissimilarity detected between mated queens and workers (P = 0.471) or non-mated queens and workers (P = 0.245).
Nest Disturbance
Fisher’s exact test showed a significant difference between mated queens and workers for brood collecting behaviour (P = 0.00905) but not for the fleeing behaviour (P = 0.0824). There was no significant difference between mated and non-mated queens for either behaviour (flee P = 0.221, collect brood P = 0.109) or between non-mated queens and workers although the non-mated queens and workers were similar in that they always fled the nest chamber (flee P = 1.00, collect brood P = 0.592).
Normal Nest Behaviour
The behavioural ranges of mated queens (blue) and non-mated queens (green) do not overlap in figure 7 while workers (red) are shown to have a wider range of behaviours overlapping with both mated and non-mated queens.
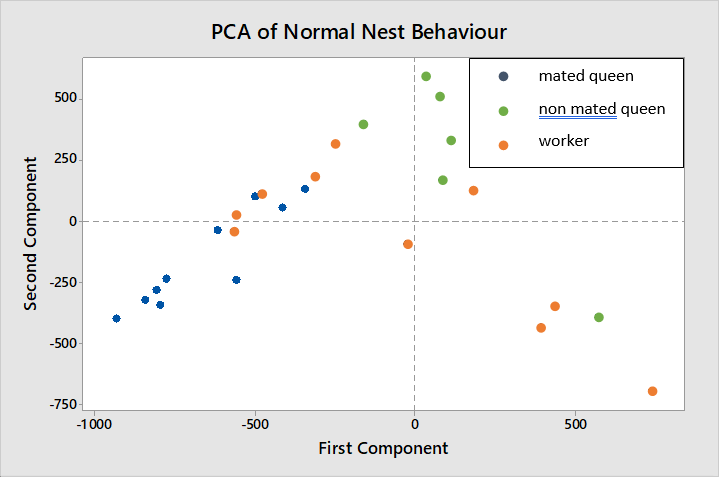
The variables having greatest weighting in the first and second components are, rest (-0.777) and forage (0.615), and move (0.737) and forage (-0.579) respectively, therefore these are the behaviours in which most variation is seen between mated queens, non-mated queens and workers.
DFA also identified non-mated queens as a distinct group, and mated queens and workers as mostly distinct groups with some overlap. All three groups were shown to form groupings based on the behavioural tropes very similar to that of their caste groups (P = 0.923). Only the activities rest, groom self, groom other and get groomed could be included as predictors as other variables either showed too high a correlation with another variable (move), or were too constant in at least one group (bully, get bullied, forage).
Correlation between behavioural differences and ovariole development
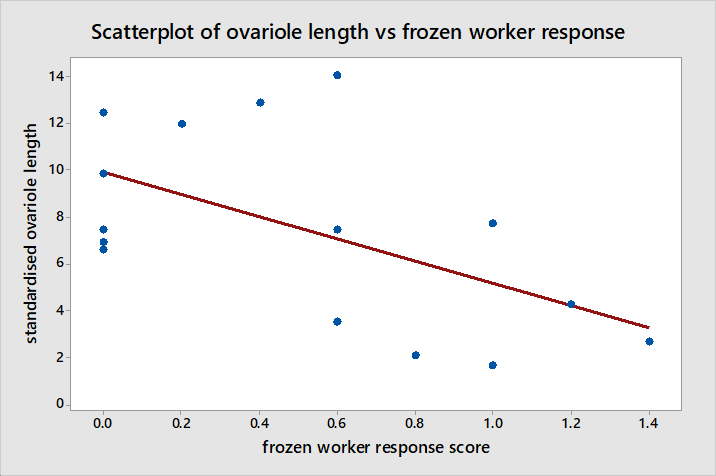
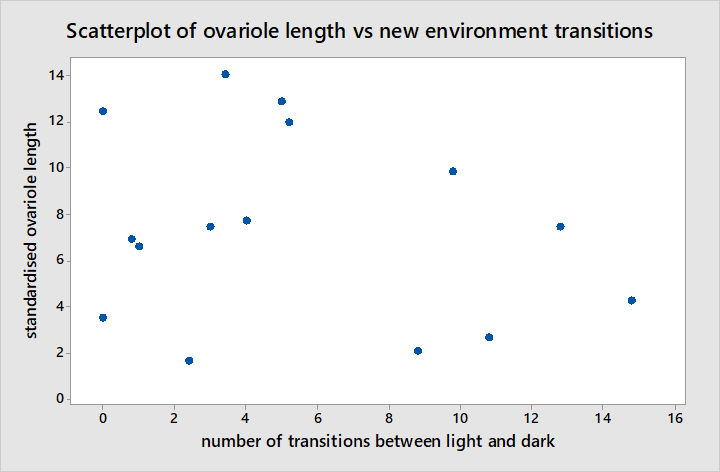
The Pearson correlation between standardised ovariole length and the response scores from interactions with frozen workers suggests there is a moderate negative correlation as illustrated in figure 8 between the two (r = -0.560, P = 0.030). Although there is a significant correlation here, this is not very strong and data points appear fairly scattered around the regression line such that it is unclear whether the change in behaviour is gradual in relation to ovariole development or whether it occurs suddenly at a given point in ovariole development. Furthermore standardised ovariole length has no significant correlation with the number of transitions made between light and dark areas during NE in figure 9 (r = -0.225, P = 0.420).
Discussion
New environment
That workers and non-mated queens made a larger number of transitions between light and shaded areas than mated queens, suggests that these castes are more active and exploratory. Such a difference in exploration has also been seen between mated and non-mated queens during spring where the exploration by virgin queens has been proposed as a behaviour which may increase mating success (Bernadou & Heinze, 2013). The similarity in behaviour from spring, when the nuptial flights occur, and autumn raises the possibility that unmated queens do not undergo a behavioural change after unsuccessful attempts to mate and retain the behavioural traits which help them to find a mate.
However the lack of this exploratory behaviour in mated queens may have hindered accurate assessment of whether they prefer light or shaded areas. Some mated queens, specifically SR.1 queens 1 and 2 and SR.4 queen 4, never moved far enough to find the shaded area so perhaps a different experimental design is needed to assess whether they have a preference for light or dark environments. For example the design used by (Bernadou & Heinze, 2013) provided and greater difference in light levels between the two available areas which were closer to where a queen was presented so that queens did not have to move as far. Using that experimental design mated queens appeared to have a slight preference for the dark area while the preference of non-mated queens showed a strong preference for the light area.
Similarly to the other mated queens SR.2 queen 2 only moved into the shaded area as the lid was being put on before timing started and did not explore outside the shaded area until the final period while other ants explored the entire perimeter of the container. Therefore although transitions between light and dark can serve as a proxy for activity or exploration in this study it would be worthwhile conducting an experiment better targeted at studying this behaviour such as that of Cole and Cheshire (1996).
Frozen worker interactions
Mated queens more often fled while non-mated queens sometimes displayed aggression though ignoring the presence of the non-nest mate individual was frequent in all castes with workers almost always ignoring the non-nest mate worker. This implies mated queens are actively defensive and virgin queens actively aggressive in comparison to the largely neutral behaviour of workers to conspecifics.
Other Leptothorax species have been shown to distinguish between nest mates and individuals of geographically close colonies by chemical cues regardless of relatedness (Hare, 1996; Stuart & Herbers, 2000). Therefore L. acervorum individuals should respond in same way to an individual from any other colony and relatedness could not have caused individuals to ignore the frozen worker. The high frequency of ignoring responses could have arisen due to workers being frozen for several days before being used in an experiment. However chemicals which related polydomous species use to distinguish their nest from another’s and would be on all individuals from a nest last several hours and even days without freezing (Alloway, 1990). Furthermore other aggression tests with live individuals have yielded similarly amicable reactions to non-nest mate conspecifics (Stuart & Herbers, 2000).
Antennal probing
Further supporting the findings from the frozen worker interactions mated queens always fled from the unfamiliar interaction with the forceps while half of non-mated queens usually displayed aggression towards the forceps. Workers tended to be less aggressive than non-mated queens but more aggressive than the mated queens. Since stroking the antennae of an individual using fine forceps is a similar interaction to the antennation which sometimes occurs between nest mates it could produce a similar response (Ito, 2005). Such antennation is mildly aggressive so it is reasonable to suppose it could induce responses like the ones seen here for example fleeing from the aggressor or giving a threat display in return. If correct this means virgin queens would react more aggressively than either other caste to any such mildly aggressive interaction in the nest.
Some studies also examined aggression behaviour during normal nest behaviour in monogynous colonies and suggested workers and virgin queens to be similar in their aggression towards nest mates, specifically towards low ranking mated queens in monogynous colonies (Felke & Buschinger, 1999; Ito, 2005; Ortius & Heinze, 1999). They also observed that dominant mated queens rarely showed any aggressive behaviour as seen here. However both the antennal probing experiment and the frozen worker interactions suggest virgin queens are more aggressive than either other caste and are not significantly similar to workers. This would mean there is greater variation in aggression between members of colonies which contain virgin queens than in those which do not. Such variation in aggression has been linked to increased productivity in colonies of the related genus Temnothorax (Modlmeier & Foitzik, 2011).
Given the limited number of spare colony boxes most individuals were tested in a box which had already contained a different individual for this experiment. This could have caused the reaction of an individual to be affected by pheromones left by previous ants in the colony box, which have been shown to be effective for over 14 hours in the case of alarm pheromones in Temnothorax rugutulus (Sasaki, Holldobler, Millar, & Pratt, 2014). However as Sasaki commented the alarm pheromone is highly volatile and may not persist as long in containers which are open and not in small enclosed spaces. By comparison pheromone trails which are laid by workers to lead nest mates to food sources and are exposed to open air are only effective for four minutes (Stuart & Moffett, 1994). Because of this it is unlikely that alarm pheromones persisted in the experimental colony boxes when they were left for more than one or two days between experiments as they often were so would not have affected the ants’ behaviours.
Nest disturbance
A nest disturbance such as was created in this experiment shows the only significant similarity between workers and virgin queens found in this study. All workers and virgin queens invariably fled the exposed nest chamber which not all mated queens did. Reproductive queens may stay within their original nest because they lack the exploratory behaviour or mobility to find a less exposed area and are waiting for workers to find a new nest site and carry them to it (Pratt, Mallon, Sumpter, & Franks, 2002). An example of such behaviour was seen when colonies were first transferred to colony boxes where queens were carried into the nest by workers along with brood items. Some mated queens were also carried back to the nest by workers when queens were put into foraging area after experiments or marking.
There was a significant difference between mated queens and workers in the number of individuals who regularly collected brood items and moved them to less exposed areas when the artificial nests were disturbed but not between non-mated queens and either of the other groups. This could suggest a behaviour in virgin queens which is an intermediate between the behaviours of mated queens and workers as seen in other polygynous species of ant (Bouchet, Peeters, Fisher, & Molet, 2013). Alternatively the view could be taken that both workers and virgin queens were seen to collect brood items while reproductive queens were never seen to collect brood items, an observation also made in other studies (Hare, 1996; Swan & Hare, 2012).
Normal nest behaviour
The behavioural tropes of mated and virgin queens do form distinctive clusters when analysed using PCA. However the data points for virgin queens more than mated queens are still quite diffuse and would produce more reliable estimate of the level of difference between the two groups if a larger sample were used. Worker data points were especially diffuse overlapping with both mated and non-mated queen tropes. This could mean that workers are more variable in their behaviour than either other caste, though the principal components attach different weightings to different behaviours so this inference only applies to the highest weighted activities, forage, rest and move. The queen castes could be more variable in their behaviour concerning other activities which would not be well represented in this PCA.
DFA identified all non-mated queens as one separate group from the other castes based on their behaviour while one worker being grouped with the majority of the mated queens and one mated queen likewise being grouped with the workers indicates that the behavioural tropes of these two castes are more similar based on the time spent on resting and grooming activities.
Both DFA and PCA indicate that non-mated queens display a larger difference in behaviour to mated queens than workers do. Furthermore while the behavioural trope of non-mated queens is more closely aligned to that of workers than it is to that of mated queens it does not fall within the behavioural range of workers completely. This suggests that a third behavioural trope distinct from that of the other two castes is represented by non-mated queens.
PCA shows that the key behavioural differences between mated and non-mated queens while in an established nest are that mated queens spend more time resting while non-mated queens spend more time moving around and leave the nest to forage more. One explanation why mated queens are less active could be partly because they have fewer interactions with other ants as they are often at the centre of a group of resting workers and also because they are less susceptible to activation from these interactions than non-mated queens and workers are (Cole & Cheshire, 1996). This effect would have been exacerbated by the shape of the artificial nest chamber which is wider than the narrow nests L. acervorum colonies inhabit inside twigs. This is because resting ants form large groups in the artificial nests where individuals at the centre are not disturbed often and there is not enough space for such large groups inside natural nests meaning resting individuals are disturbed more often in natural nests. However they also seem to move more slowly even when active as observed during the new environment experiment. Additionally when mated queens were disturbed by interactions from other individuals they rarely performed other activities in the nest which the other two castes did such as grooming others and themselves.
Some studies have found evidence that the time spent on other nest activities may also differ between mated and non-mated queens more subtly than activities such as resting and foraging so have not been as well represented in this study. Some papers suggest non-reproductive queens are groomed less than reproducing queens however this is an observation from monogynous colonies which may only apply to subordinate mated queens not virgin queens (Gill & Hammond, 2011a, 2011c). Ito (2005) further suggests that virgin queens are similar to workers in that they take part in bullying and expelling low ranking mated queens in monogynous colonies of L. acervorum as previously stated, which dominant mated queens do not. Moreover virgin queens are not bullied unlike the mated subordinate queens in monogynous colonies (Felke & Buschinger, 1999). Since such behaviour is uncommon in polygynous colonies this could not be tested in this paper but is supported by other studies on colonies of L. acervorum.
The wire marking method used may have been causing individuals to be groomed more or groom themselves more in an attempt to remove the wires. However since all the queens were marked and would have been similarly affected, and grooming did not present a major contribution to the first two components of PCA, any affect it had on the results is negligible. Also the normal nest behaviour experiment does not take into account whether the focus of the sample is bullying or grooming another alone or if the focus grooms or bullies an individual at the same time as multiple other ants are grooming or bullying the same individual. Multiple ants interacting with a single individual was a very rare occurrence though so would be unlikely to identify a difference between castes in a study of this size.
Correlation of ovarian development with behaviours
As the behaviour of queens changes with ovariole development it is clear that this behavioural change is linked to mating and reproduction in the same way as the developing ovaries. However the exact cause of such changes is less clear.
The correlation is too weak to suggest a direct link between ovariole development and behavioural changes thus it is more likely that behavioural changes result from other physiological cues triggered by mating than from cues triggered directly by ovariole development. The correlation is also too weak with data points too dispersed to offer any insight as to whether behavioural changes develop gradually or suddenly at a given point in relation to ovariole development. It is unlikely that the correlation appears weaker here than it is because of reasons other than small sample size since it mating takes place in summer and ovariole development occurs in spring so the behaviours of recently mated queens would not have been changing during data collection (Felke & Buschinger, 1999; Gill & Hammond, 2011c). Additionally the fact that mated status was not known until dissections were performed eliminates the possibility of bias during data collection.
Some papers have suggested spermathecal stretching causing the spermathecal to release hormones, or chemicals in secretions of male accessory glands during mating as the cause of behavioural changes in hymenopteran queens after mating (Oppelt et al., 2010; Richard, Tarpy, & Grozinger, 2007). However behavioural changes are more closely linked to onset of reproduction than mating since functionally monogynous colonies have mated queens which do not undergo many of the physical and behavioural changes. Therefore there must be an intermediate step allowing regulation if spermathecal stretching for example were the ultimate cause of changes. The physiological and behavioural changes after mating would then happen in two stages, the first would start straight after mating when surface chemicals identifying the queen as mated are produced (Ortius & Heinze, 1999), the second stage would occur around the time of reproductive development when changes in gene expression and brain volume have been observed and associated with some of the behavioural changes from virgin to reproductive queens (Julian & Gronenberg, 2002; Wurm, Wang, & Keller, 2010).
Workers have also been shown to undergo a behavioural change associated with reproductive development although the changes are slightly different to those which occur in queens. Workers with more developed ovaries tend to forage less and take part in brood care less similarly to reproductive queens, however where the reproductive queens were more defensive and explored less workers become more aggressive and explorative (Friend & Bourke, 2014; Kuhbandner et al., 2014). This change in behaviour associated with ovariole development has been linked to the presence of endocrines produced by the ovarioles such as ecdysteroid hormones. The same mechanism could be responsible for the behavioural changes in queens at the onset of reproductive development however no studies have looked for such a mechanism in Leptothorax queens thus far.
Future research
It has been established that unmated queens are dealated by workers to prevent them mating thereby limiting the resource cost on the colony incurred by reproducing queens (Hammond et al., 2003; Trettin et al., 2014). It is also apparent that these delate virgin queens perform many activities which contribute to the normal functioning of the colony which workers also perform. However since the virgin queens have a behavioural trope subtly different to workers and significantly different to mated queens, the complete scope of the functional role this behavioural trope plays in a colony is still unclear. To investigate what this role could be future studies into the extent of the behavioural differences between the three castes and the effect on the colony are necessary.
While the small sample size has shown that the difference between the behaviours of each caste are substantial to produce sufficient statistical power to identify significant differences between the castes, it does mean that subtler differences are not apparent such as those between virgin queens and workers. To better gauge the extent of the behavioural differences between castes and how their roles in a colony differ, further studies of aggression, normal nest behaviour, and light or shade preferences are needed with much larger samples. As previously stated a more directed study of exploration and activity could also be made.
Following on from the proposal that the virgin queen behavioural trope may change into the reproductive mated queen behavioural trope in two stages further experiments could be made looking at which stage the functional role of a queen changes at. This could be done by looking at which trait, mated status or reproductive status, best predicts which behavioural trope a queen has. Since there can be egg laying virgin queens in L. acervorum, queens can be grouped by reproductive status or by mated status then used to determine what causes the behavioural change based on whether groups show greater difference when grouped by mated status or by whether they lay eggs. Alternatively future studies could look further into the causes of the behavioural changes focussing on which genes expressed during the behavioural change stimulate reduction in brain volume and what causes the expression of these genes for example. In addition the effect of ecdysteroids on virgin queens could be examined by measuring the correlation of ecdysteroid production of behavioural change or brain volume (Brent & Dolezal, 2009), or by measuring the effect of artificially raised ecdysteroid levels or ectopic production of the hormone in virgin queens.
Future studies could also examine the benefits to colonies of having more unmated dealate queens in terms of eggs laid for example. Since unreproductive mated queens show similar behaviour to unmated queens it is possible that they benefit polygynous colonies in the same way that subordinate mated queens benefit monogynous colonies (Heinze & Oberstadt, 2003). Though the mechanism through which subordinate queens benefit colonies is not clear, it has been shown that reproductive queens with subordinate queens produce more offspring than reproductive queens which have no subordinate queens. If the third behavioural trope represented by virgin queens does increase colony productivity, the increased variation in aggression between group members over a purely worker based workforce could offer one explanation for this increase in productivity (Modlmeier & Foitzik, 2011). However there could be other reasons such as greater independent activity in virgin queens which may be highlighted by further studies into differences between non-mated queens and workers.
Alternatively this third behavioural trope in the colony could be a retention of alate queen behaviours in dealate non-mated queens, which does not increase the productivity of the colony significantly but neither decreases it. In such a scenario expelling unmated queens would not benefit the colony but if kept in the colony these virgin queens could act as insurance, retaining behaviours which could help them find a mate if the colony were to lose its existing mated queens. Since reproductive queens do not flee when the nest is disturbed this is entirely possible. Dealate queens have been found to crawl around underneath the nuptial flights of conspecifics and seek higher points from which to attract males (Bernadou & Heinze, 2013; Felke & Buschinger, 1999).
It is possible that the behaviour and therefore role of virgin queens differs between populations however in the case of another behavioural difference monogynous and polygynous colonies it has been suggested that there is no actual difference in the behavioural tropes of queens from each population. The more aggressive behaviour of mated queens in the Japanese and Spanish colonies compared to the passive and defensive reactions shown by English mated queens could be explained by the behavioural change after mating not being high aggression to low aggression in some populations and high aggression in others, but high aggression to an aggression behaviour which is dependent on environmental factors such as food availability or the queen-worker ratio (Ito, 2005; Trettin et al., 2014). This directly contradicts the findings of previous papers which indicate that polymorphisms in social organisation between populations are stable (Gill et al., 2009). This could be because purely monogynous populations have lost such plasticity or because the change from monogyny to polygyny is much slower. Because of this dispute it cannot be ruled out that virgin queen behaviour as well as mated queen behaviour differs between populations and that virgin queens play a role closer to that of workers in monogynous colonies of L. acervorum due to their involvement in influencing the reproduction of mated queens. To resolve this a separate study would have to be conducted on monogynous colonies.
Conclusion
The behavioural differences and similarities between the three castes examined here are situational in that they vary in level of difference with different situations. The key differences between mated and unmated queens found here were that mated queens rested more and moved slower, did not perform brood care behaviours or flee in search of a safer place if the nest was disturbed. They were less exploratory and generally less aggressive preferring to flee rather than fight, indicating that mated queens had a much reduced behavioural repertoire compared to non-mated queens. The notable similarities found between non-mated queens and workers were that every member of these two castes all fled the disturbed nest chamber and collected brood items, as well as all grooming brood items when in an undisturbed nest.
Furthermore although PCA of normal nest behaviour suggests there is some similarity between worker and virgin queen behaviour, virgin queens of L. acervorum do not always behave as one might expect a worker to. Thus non-mated queens may represent a third behavioural trope which in some cases differs to that of mated queens more than the behavioural trope of workers differs to mated queens, such as with aggression, and other times differs less such as with exploration and brood care. Therefore the role of virgin queens in polygynous colonies of L. acervorum is not simply to act as a worker and they may perform some additional function. Further larger scale studies are needed to identify what this additional function could be, the level of behavioural differences and similarities between these castes and what physiological changes after mating trigger the behavioural changes in queens after mating, but the findings of these experiments may offer a solid basis for future research.
References
Alloway, T. M., Hodgeson, Sandra. (1990). Nest recognition in the ant Leptothorax ambiguus Emery (Hymenoptera: Formicidae). Psyche, 97(3-4), 175-180.
Bernadou, A., & Heinze, J. (2013). Mating-Associated Changes in the Behaviour of Leptothorax gredleri Ant Queens. Ethology, 119(8), 634-643. doi: 10.1111/eth.12103
Boi, S., Couzin, I. D., Del Buono, N., Franks, N. R., & Britton, N. F. (1999). Coupled oscillators and activity waves in ant colonies. Proceedings of the Royal Society B-Biological Sciences, 266(1417), 371-378.
Bouchet, D. C., Peeters, C., Fisher, B. L., & Molet, M. (2013). Both female castes contribute to colony emigration in the polygynous ant Mystrium oberthueri. Ecological Entomology, 38(4), 408-417. doi: 10.1111/een.12033
Bourke, A. F. G. (1991). Queen behavior, reproduction and egg cannibalism in multiple-queen colonies of the ant Leptothorax acervorum. Animal Behaviour, 42(2), 295-310.
Bower, K. M. (2003). When To Use Fisher’s Exact Test. ASQ Six Sigma Forum Magazine, 2(4).
Brent, C., & Dolezal, A. (2009). Ant ecdysteroid extraction and radioimmunoassay. Cold Spring Harbor protocols, 2009(7), pdb.prot5247. doi: 10.1101/pdb.prot5247
Cole, B. J., & Cheshire, D. (1996). Mobile cellular automata models of ant behavior: Movement activity of Leptothorax allardycei. American Naturalist, 148(1), 1-15. doi: 10.1086/285908
Cole, B. J., & Hoeg, L. (1996). The influence of brood type on activity cycles in Leptothorax allardycei (Hymenoptera: Faruicidae). Journal of Insect Behavior, 9(4), 539-547. doi: 10.1007/bf02213878
Coston, D. J., Gill, R. J., & Hammond, R. L. (2011). No evidence of volatile chemicals regulating reproduction in a multiple queen ant. Naturwissenschaften, 98(7), 625-629. doi: 10.1007/s00114-011-0801-4
Eliyahu, D., Ross, K. G., Haight, K. L., Keller, L., & Liebig, J. (2011). Venom Alkaloid and Cuticular Hydrocarbon Profiles Are Associated with Social Organization, Queen Fertility Status, and Queen Genotype in the Fire Ant Solenopsis invicta. Journal of Chemical Ecology, 37(11), 1242-1254. doi: 10.1007/s10886-011-0037-y
Felke, M., & Buschinger, A. (1999). Social organization, reproductive behavior and ecology of Leptothorax acervorum (Hymenoptera, Formicidae) from the Sierra de Albarracin in central Spain. Insectes Sociaux, 46(1), 84-91. doi: 10.1007/s000400050117
Friend, L. A., & Bourke, A. F. G. (2012). Absence of Within-Colony Kin Discrimination in a Multiple-Queen Ant, Leptothorax acervorum. Ethology, 118(12), 1182-1190. doi: 10.1111/eth.12024
Friend, L. A., & Bourke, A. F. G. (2014). Workers respond to unequal likelihood of future reproductive opportunities in an ant. Animal Behaviour, 97, 165-176. doi: 10.1016/j.anbehav.2014.09.013
Gill, R. J., Arce, A., Keller, L., & Hammond, R. L. (2009). Polymorphic social organization in an ant. Proceedings of the Royal Society B-Biological Sciences, 276(1677), 4423-4431. doi: 10.1098/rspb.2009.1408
Gill, R. J., & Hammond, R. L. (2011a). Workers determine queen inheritance of reproduction in a functionally monogynous ant population. Animal Behaviour, 82(1), 119-129. doi: 10.1016/j.anbehav.2011.04.006
Gill, R. J., & Hammond, R. L. (2011c). Workers influence royal reproduction. Proceedings of the Royal Society B-Biological Sciences, 278(1711), 1524-1531. doi: 10.1098/rspb.2010.1774
Hammond, R. L., Bruford, M. W., & Bourke, A. F. (2003). Male parentage does not vary with colony kin structure in a multiple-queen ant. J Evol Biol, 16(3), 446-455.
Hammond, R. L., Bruford, M. W., & Bourke, A. F. G. (2006). A test of reproductive skew models in a field population of a multiple-queen ant. Behavioral Ecology and Sociobiology, 61(2), 265-275. doi: 10.1007/s00265-006-0257-2
Hare, J. F. (1996). Discrimination of nestmate larvae by the ant Leptothorax longispinosus. Canadian Journal of Zoology, 74(11), 2055-2061. doi: 10.1139/z96-233
Hatcher, M. J., Tofts, C., & Franks, N. R. (1992). MUTUAL EXCLUSION AS A MECHANISM FOR INFORMATION EXCHANGE WITHIN ANT NESTS. Naturwissenschaften, 79(1), 32-34. doi: 10.1007/bf01132279
Heinze, J., & Buschinger, A. (1987). QUEEN POLYMORPHISM IN A NONPARASITIC LEPTOTHORAX SPECIES (HYMENOPTERA, FORMICIDAE). Insectes Sociaux, 34(1), 28-43. doi: 10.1007/bf02224205
Heinze, J., Holldobler, B., & Trenkle, S. (1995). REPRODUCTIVE-BEHAVIOR OF THE ANT LEPTOTHORAX (DICHOTHORAX) PERGANDEI. Insectes Sociaux, 42(3), 309-315. doi: 10.1007/bf01240425
Heinze, J., Lipski, N., Schlehmeyer, K., & Holldobler, B. (1995). Colony structure and reproduction in the ant, Leptothorax acervorum. Behavioral Ecology, 6(4), 359-367. doi: 10.1093/beheco/6.4.359
Heinze, J., & Oberstadt, B. (2003). Costs and benefits of subordinate queens in colonies of the ant, Leptothorax gredleri. Naturwissenschaften, 90(11), 513-516. doi: 10.1007/s00114-003-0467-7
Ito, F. (2005). Mechanisms regulating functional monogyny in a Japanese population of Leptothorax acervorum (Hymenoptera, Formicidae): dominance hierarchy and preferential egg cannibalism. Belgian Journal of Zoology, 135(1), 3-8.
Julian, G. E., & Gronenberg, W. (2002). Reduction of brain volume correlates with behavioral changes in queen ants. Brain Behavior and Evolution, 60(3), 152-164. doi: 10.1159/000065936
Kuhbandner, S., Modlmeier, A. P., & Foitzik, S. (2014). Age and ovarian development are related to worker personality and task allocation in the ant Leptothorax acervorum. Current Zoology, 60(3), 392-400.
Minitab Inc. (2010). Minitab 17 Statistical Software. State College, Pennsylvania. Retrieved from www.minitab.com
Modlmeier, A. P., & Foitzik, S. (2011). Productivity increases with variation in aggression among group members in Temnothorax ants. Behavioral Ecology, 22(5), 1026-1032. doi: 10.1093/beheco/arr086
Oberstadt, B., & Heinze, J. (2003). Mating biology and population structure of the ant, Leptothorax gredleri. Insectes Sociaux, 50(4), 340-345. doi: 10.1007/s00040-003-0681-5
Oppelt, A., & Heinze, J. (2009). Mating is associated with immediate changes of the hydrocarbon profile of Leptothorax gredleri ant queens. Journal of Insect Physiology, 55(7), 624-628. doi: 10.1016/j.jinsphys.2009.03.010
Oppelt, A., Humann, F. C., Fuessl, M., Azevedo, S. V., Antonio, D. S. M., Heinze, J., & Hartfelder, K. (2010). Suppression subtractive hybridization analysis reveals expression of conserved and novel genes in male accessory glands of the ant Leptothorax gredleri. Bmc Evolutionary Biology, 10. doi: 10.1186/1471-2148-10-273
Ortius, D., & Heinze, J. (1999). Fertility signaling in queens of a North American ant. Behavioral Ecology and Sociobiology, 45(2), 151-159. doi: 10.1007/s002650050548
Pratt, S. C., Mallon, E. B., Sumpter, D. J. T., & Franks, N. R. (2002). Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behavioral Ecology and Sociobiology, 52(2), 117-127. doi: 10.1007/s00265-002-0487-x
Rasband, W. S. (1997-2014). ImageJ. Bethesda, Maryland, USA: U. S. National Institutes of Health. Retrieved from http://imagej.nih.gov/ij/
Richard, F. J., Tarpy, D. R., & Grozinger, C. M. (2007). Effects of Insemination Quantity on Honey Bee Queen Physiology. Plos One, 2(10). doi: 10.1371/journal.pone.0000980
Roulston, T. H., Buczkowski, G., & Silverman, J. (2003). Nestmate discrimination in ants: effect of bioassay on aggressive behavior. Insectes Sociaux, 50(2), 151-159. doi: 10.1007/s00040-003-0624-1
Ruppell, O., Schaffler, L., & Holldobler, B. (2002). Lack of plasticity in the behavior of queens of the ant Leptothorax rugatulus emery (Formicidae : Hymenoptera). Journal of Insect Behavior, 15(3), 447-454. doi: 10.1023/a:1016277511957
Sasaki, T., Holldobler, B., Millar, J. G., & Pratt, S. C. (2014). A context-dependent alarm signal in the ant Temnothorax rugatulus. Journal of Experimental Biology, 217(18), 3229-3236. doi: 10.1242/jeb.106849
Schrempf, A., Cremer, S., & Heinze, J. (2011). Social influence on age and reproduction: reduced lifespan and fecundity in multi-queen ant colonies. Journal of Evolutionary Biology, 24(7), 1455-1461. doi: 10.1111/j.1420-9101.2011.02278.x
Stuart, R. J., & Herbers, J. M. (2000). Nest mate recognition in ants with complex colonies: within- and between-population variation. Behavioral Ecology, 11(6), 676-685. doi: 10.1093/beheco/11.6.676
Stuart, R. J., & Moffett, M. W. (1994). RECRUITMENT COMMUNICATION AND PHEROMONE TRAILS IN THE NEOTROPICAL ANTS, LEPTOTHORAX (NESOMYRMEX) SPININODIS AND L (N) ECHINATINODIS. Experientia, 50(9), 850-852. doi: 10.1007/bf01956470
Swan, D. C., & Hare, J. F. (2012). Larval recognition by Temnothorax longispinosus and T-ambiguus hosts of the slave-making ant Protomognathus americanus revisited: colony-level referent ensures conspecific preference. Insectes Sociaux, 59(4), 511-517. doi: 10.1007/s00040-012-0245-7
Thomas M. Alloway, A. B., Mary Talbot, Robin Stuart, and Cynthia Thomas. (1982). Polygyny and Polydomy in Three North American Species of the Ant Genus Leptothorax Mayr (Hymenoptera: Formicidae). Psyche, 89(3-4), 249-274.
Trettin, J., Seyferth, T., & Heinze, J. (2014). Behavioral Plasticity in Ant Queens: Environmental Manipulation Induces Aggression among Normally Peaceful Queens in the Socially Polymorphic Ant Leptothorax acervorum. Plos One, 9(4). doi: 10.1371/journal.pone.0095153
Wurm, Y., Wang, J., & Keller, L. (2010). Changes in reproductive roles are associated with changes in gene expression in fire ant queens. Molecular Ecology, 19(6), 1200-1211. doi: 10.1111/j.1365-294X.2010.04561.x XL Imaging Ltd. (2006). Xli Cap. Swansea.